Research on the Construction of CuFeS2/ Bi2O3 Composite Materials and Degradation of Bisphenol A by Activating PMS
Abstract
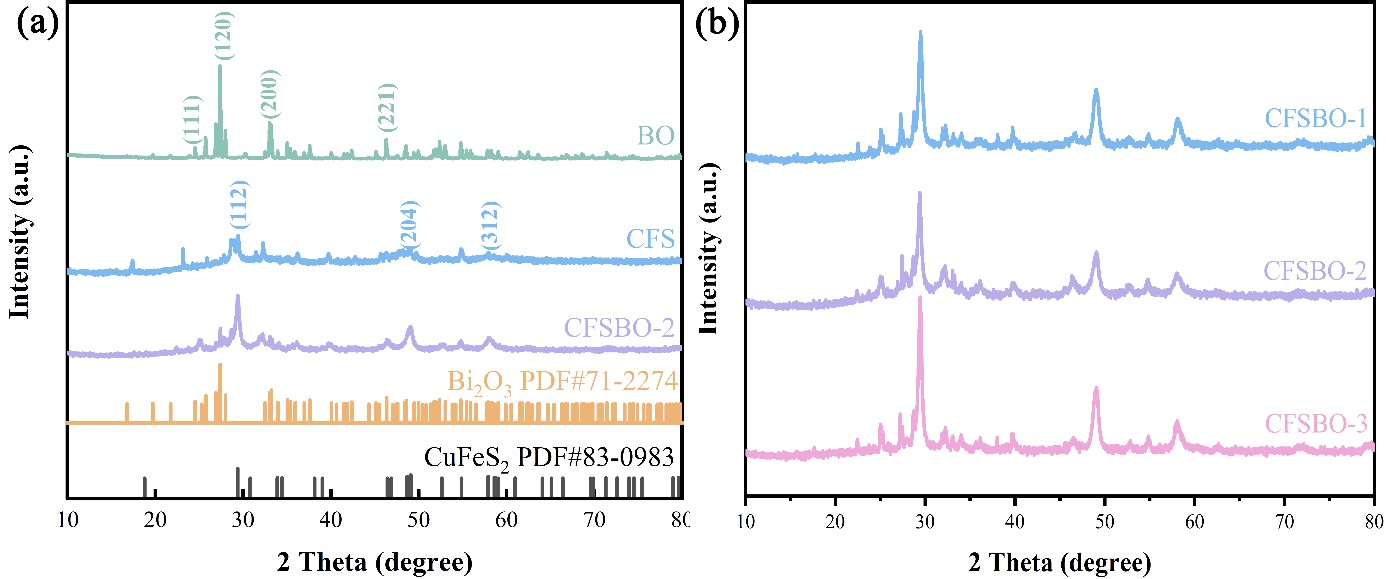
Bisphenol A (BPA) is harmful to human health. Advanced oxidation technologies using peroxymonosulfate (PMS) can effectively remove organic pollutants. Among these technologies, bimetallic sulfides stand out for their excellent activation ability. This study focuses on CuFeS2, and CuFeS2/Bi2O3 catalysts were prepared using a hydrothermal method. The CFSBO-2/PMS system can degrade up to 84.06% of BPA in just 30 seconds. XRD analysis shows that compared to the original CFS, the CFSBO catalyst significantly enhances the intensity of diffraction peaks, indicating improved crystallinity. The system maintains high degradation efficiency as the pH increases from 3.6 to 9.0, suggesting that the catalyst is highly adaptable to different water treatment conditions. The main active species generated are SO4−•, •OH, and 1O2. PMS activation in this system is driven by the redox cycles of Cu+/Cu2+, Fe2+/Fe3+, and Bi3+/Bi5+.
References
[2] Li, Y., Zhu, C., Chen, L., & et al. (2023). Self-driven degradation of TC-HCl by CuFe2O4/Bi2O3 activated peroxymonosulfate. Chemical Engineering Journal, 473, 145282. https://doi.org/10.1016/j.cej.2023.145282
[3] Girma, W. M., Tzing, S.-H., Tseng, P.-J., & et al. (2018). Synthesis of cisplatin(IV) prodrug-tethered CuFeS2 nanoparticles in tumor-targeted chemotherapy and photothermal therapy. ACS Applied Materials & Interfaces, 10(5), 4590–4602. https://doi.org/10.1021/acsami.7b16108
[4] Fan, T.-E., Tang, X., Liu, S.-M. (2022). CuFeS2 nanosheets assembled into honeycomb-like microspheres as stable high-capacity anodes for sodium-ion batteries. ACS Applied Nano Materials, 5(8), 10392–10398. https://doi.org/10.1021/acsanm.2c01745
[5] Fang, L., Zhang, D., Chen, H., & et al. (2024). Efficient removal of moxifloxacin through PMS activation by CuFeS2/MXene. Environmental Science and Pollution Research, 31(32), 45353–45369. https://doi.org/10.1007/s11356-024-34123-9
[6] Wang, H., Liao, B., Hu, M., & et al. (2022). Heterogeneous activation of peroxymonosulfate by natural chalcopyrite for efficient remediation of groundwater polluted by aged landfill leachate. Applied Catalysis B: Environmental, 300, 120744. https://doi.org/10.1016/j.apcatb.2021.120744
[7] Jia, Y., Yang, K., Zhang, Z., & et al. (2023). Heterogeneous activation of peroxymonosulfate by magnetic hybrid CuFe2O4@N-rGO for excellent sulfamethoxazole degradation: Interaction of CuFe2O4 with N-rGO and synergistic catalytic mechanism. Chemosphere, 313, 137392. https://doi.org/10.1016/j.chemosphere.2022.137392
[8] Li, H., Yang, C., He, Y., & et al. (2023). Oxygen vacancies facilitated photocatalytic detoxification of three typical contaminants over graphene oxide surface embellished BiOCl photocatalysts. Advanced Powder Technology, 34(3), 103971. https://doi.org/10.1016/j.apt.2023.103971
[9] De Souza, R. A., & Mueller, D. N. (2021). Electrochemical methods for determining ionic charge in solids. Nature Materials, 20(4), 443–446. https://doi.org/10.1038/s41563-021-00947-7
[10] Walsh, A., Sokol, A. A., Buckeridge, J., & et al. (2018). Oxidation states and ionicity. Nature Materials, 17(11), 958–964. https://doi.org/10.1038/s41563-018-0165-7
[11] Cheng, D., Yuan, S., Liao, P., & et al. (2016). Oxidizing impact induced by mackinawite (FeS) nanoparticles at oxic conditions due to production of hydroxyl radicals. Environmental Science & Technology, 50(21), 11646–11653. https://doi.org/10.1021/acs.est.6b03492
[12] Ren, X., An, J., Ding, S., & et al. (2025). High efficiency degradation of RhB by MIL-88A(Fe)/MoS2 activated persulfate and its mechanism. Journal of Solid State Chemistry, 341, 125027. https://doi.org/10.1016/j.jssc.2025.125027
[13] Liu, N., Huang, W., Zhang, X., & et al. (2018). Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Applied Catalysis B: Environmental, 221, 119–128. https://doi.org/10.1016/j.apcatb.2017.08.072
[14] Ding, S., Ren, X., Chen, R., & et al. (2023). Efficient degradation of phenol by 1T/2H-MoS2 /CuFe2O4 activated peroxymonosulfate and mechanism research. Applied Surface Science, 612, 155931. https://doi.org/10.1016/j.apsusc.2022.155931
[15] Li, F., Yuan, C., Niu, Y., & et al. (2024). Cobalt/iron bimetallic oxide coated with graphitized nitrogen-doped carbon (Fe2O3-CoO@NC) derived from cobalt/iron solid complex as peroxymonosulfate (PMS) activator for efficient bensulfuron-methyl degradation. Environmental Research, 263, 120249. https://doi.org/10.1016/j.envres.2024.120249
[16] Wang, D., Suo, M., Lai, S., & et al. (2023). Photoinduced acceleration of Fe3+/Fe2+ cycle in heterogeneous FeNi-MOFs to boost peroxodisulfate activation for organic pollutant degradation. Applied Catalysis B: Environmental, 321, 122054. https://doi.org/10.1016/j.apcatb.2022.122054
[17] Wang, B., Qian, K., Yang, W., & et al. (2023). ZnFe2O4/BiVO4 Z-scheme heterojunction for efficient visible-light photocatalytic degradation of ciprofloxacin. Frontiers of Chemical Science and Engineering, 17(11), 1728–1740. https://doi.org/10.1007/s11705-023-2322-z
[18] Zhang, Y., Guo, F., Di, J., & et al. (2024). Strain-induced surface interface dual polarization constructs PML-Cu/Bi12O17Br2 high-density active sites for CO2 photoreduction. Nano-Micro Letters, 16(1), 90. https://doi.org/10.1007/s40820-023-01276-6
[19] Zhang, J., Zhou, Y., Fang, Y., & et al. (2024). Chalcopyrite functionalized ceramic membrane for micropollutants removal and membrane fouling control via peroxymonosulfate activation: The synergy of nanoconfinement effect and interface interaction. Journal of Colloid and Interface Science, 658, 714–727. https://doi.org/10.1016/j.jcis.2023.12.093
[20] Jing, J., Pervez, M. N., Sun, P., & et al. (2021). Highly efficient removal of bisphenol A by a novel Co-doped LaFeO3 perovskite/PMS system in salinity water. Science of The Total Environment, 801, 149490. https://doi.org/10.1016/j.scitotenv.2021.149490
[21] Ni, T., Yang, Z., Zhang, H., & et al. (2022). Peroxymonosulfate activation by Co3O4/SnO2 for efficient degradation of ofloxacin under visible light. Journal of Colloid and Interface Science, 615, 650–662. https://doi.org/10.1016/j.jcis.2022.02.001
[22] Guo, T., Jiang, L., Wang, K., & et al. (2021). Efficient persulfate activation by hematite nanocrystals for degradation of organic pollutants under visible light irradiation: Facet-dependent catalytic performance and degradation mechanism. Applied Catalysis B: Environmental, 286, 119883. https://doi.org/10.1016/j.apcatb.2021.119883
[23] Qian, J., Zhang, Y., Chen, Z., & et al. (2023). Sulfur-decorated Fe/C composite synthesized from MIL-88A(Fe) for peroxymonosulfate activation towards tetracycline degradation: Multiple active sites and non-radical pathway dominated mechanism. Journal of Environmental Management, 344, 118440. https://doi.org/10.1016/j.jenvman.2023.118440
[24] Zhang, X., Zhang, Y., Tian, J., & et al. (2024). Generating 1O2 and CoIV=O through efficient peroxymonosulfate activation by ZnCo2O4 nanosheets for pollutant control. Nano Research, 17(9), 8025–8035. https://doi.org/10.1007/s12274-024-6689-2
[25] Tsitonaki, A., Petri, B., Crimi, M., & et al. (2010). In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Critical Reviews in Environmental Science and Technology, 40(1), 55–91. https://doi.org/10.1080/10643380802209716
[26] Li, Y., Liu, L., Li, W., & et al. (2021). Simultaneously rapid degradation of phenylphosphonic acid and efficient adsorption of released phosphate in the system of peroxymonosulfate (PMS) and Co3O4-La2O2CO3/C derived from MOFs. Journal of Environmental Chemical Engineering, 9(6), 106332. https://doi.org/10.1016/j.jece.2021.106332
[27] Zhao, Y., Wang, H., Li, X., & et al. (2021). Recovery of CuO/C catalyst from spent anode material in battery to activate peroxymonosulfate for refractory organic contaminants degradation. Journal of Hazardous Materials, 420, 126552. https://doi.org/10.1016/j.jhazmat.2021.126552
[28] Li, X., Wang, S., Xu, B., & et al. (2022). MOF etching-induced Co-doped hollow carbon nitride catalyst for efficient removal of antibiotic contaminants by enhanced perxymonosulfate activation. Chemical Engineering Journal, 441, 136074. https://doi.org/10.1016/j.cej.2022.136074
[29] Liu, J., Zhou, J., Ding, Z., & et al. (2017). Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrasonics Sonochemistry, 34, 953–959. https://doi.org/10.1016/j.ultsonch.2016.07.018
[30] Golshan, M., Kakavandi, B., Ahmadi, M., & et al. (2018). Photocatalytic activation of peroxymonosulfate by TiO2 anchored on copper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. Journal of Hazardous Materials, 359, 325–337. https://doi.org/10.1016/j.jhazmat.2018.07.033
[31] Hu, L., Zhang, G., Liu, M., & et al. (2018). Enhanced degradation of bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix. Chemical Engineering Journal, 338, 300–310. https://doi.org/10.1016/j.cej.2018.01.013
[32] Liu, Y., Guo, H., Zhang, Y., & et al. (2019). Fe@C carbonized resin for peroxymonosulfate activation and bisphenol S degradation. Environmental Pollution, 252, 1042–1050. https://doi.org/10.1016/j.envpol.2019.06.018
[33] Li, N., Wang, Y., Cheng, X., & et al. (2022). Influences and mechanisms of phosphate ions onto persulfate activation and organic degradation in water treatment: A review. Water Research, 222, 118896. https://doi.org/10.1016/j.watres.2022.118896
[34] Fu, H., Ma, S., Zhao, P., & et al. (2019). Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism. Chemical Engineering Journal, 360, 157–170. https://doi.org/10.1016/j.cej.2018.11.197
[35] Zhao, Y., Song, M., Cao, Q., & et al. (2020). The superoxide radicals’ production via persulfate activated with CuFe2O4@Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil. Journal of Hazardous Materials, 400, 122887. https://doi.org/10.1016/j.jhazmat.2020.122887
[36] Sun, X., Xu, D., Dai, P., & et al. (2020). Efficient degradation of methyl orange in water via both radical and non-radical pathways using Fe-Co bimetal-doped MCM-41 as peroxymonosulfate activator. Chemical Engineering Journal, 402, 125881. https://doi.org/10.1016/j.cej.2020.125881
[37] Guan, Y.-H., Ma, J., Ren, Y.-M., & et al. (2013). Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Research, 47(14), 5431–5438. https://doi.org/10.1016/j.watres.2013.06.023
[38] Ding, Y., Zhu, L., Wang, N., & et al. (2013). Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Applied Catalysis B: Environmental, 129, 153–162. https://doi.org/10.1016/j.apcatb.2012.09.015
[39] Wang, N., Liu, Y., Wu, C., & et al. (2022). SnO2 shells-induced rich Co2+ sites and oxygen vacancies in FexCo3-xO4 nanocubes: Enhanced peroxymonosulfate activation performance for water remediation. Chemical Engineering Journal, 439, 135682. https://doi.org/10.1016/j.cej.2022.135682

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
























