Formulation and Quality Optimization of Effervescent Tablet of Glipizide: An Approach to Comfort Anti-Diabetic Medication
Abstract
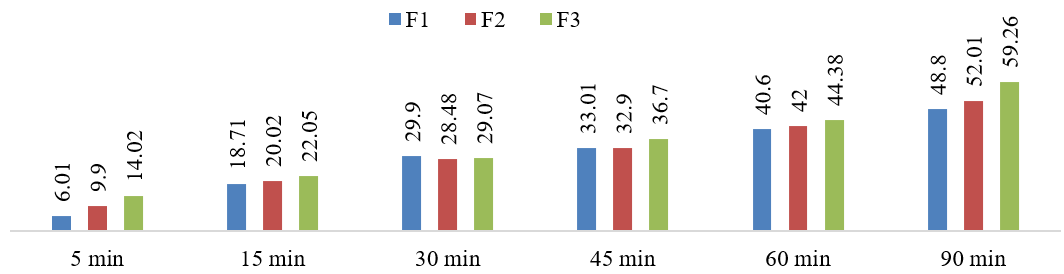
The present study is targeted to formulate and prepare effervescent tablets of Glipizide to provide more elegancy, comfortability, and improved pharmacokinetics in diabetic treatment than the conventional dosage. Three formulations (F1, F2, and F3) of the effervescent tablet of Glipizide (5mg) were formulated with different amounts and ratios of excipients. By wet granulation technique, 60 tablets for every formulation were prepared with a weight of 700mg per tablet. Then, the features of both granules and tablets were evaluated by published methods. The angle of repose, Hausner ratio, Carr's index, Loss on drying (LOD), and Moisture Content (MC) used to measure granules property successfully proved right follow ability and compressibility. In contrast, physical and drug content related investigation failed to determine the perfectness of all three formulations. Friability on the formulations was around 0.70%, indicating the expected USP limit of friability (0.5 to 1%). The mean disintegration time of the formulations was from 95s to 105s. The tablet potency assay found 95.20% for F1, 88.80% for F2, and 97.40% for F3. The dissolution pattern of the drug followed a linear relationship with time. After one and a half hours, the highest amount of 59.20% cumulative dissolution was determined for F3 that revealed its strategic improvement of the drug solubility. As Glipizide is a poorly water-soluble drug, the effervescent tablet might mitigate disintegration and dissolution-related limitations and, consequently, enhance the drug's bioavailability.
References
Agrawal, A. G., Kumar, A., & Gide P. S. (2015). Self emulsifying drug delivery system for enhanced solubility and dissolution of Glipizide. Colloid Surf B, 126, 553-560. https://doi.org/10.1016/j.colsurfb.2014.11.022
Agyilirah, G. A., Green, M., & Banker, G. S. (1991). Evaluation of the gastric retention properties of a cross-linked polymer-coated tablet versus those of a non-disintegrating tablet. International Journal of Pharmaceutics, 75(2-3), 241-247. https://doi.org/10.1016/0378-5173(91)90198-W
Al-Hashemi, H. M. B., & Al-Amoudi, O. S. B. (2018). A review on the angle of repose of granular materials. Powder Technology, 330, 397-417. https://doi.org/10.1016/j.powtec.2018.02.003
Ashish, P., Mishra, P., Main, P., Harsoliya, M., & Agrawal, S. (2011). A review on-recent advancement in the development of rapid disintegrating tablet. Int J Life Sci Pharm Res, 1, 7-16.
Aslani, A., & Fattahi, F. (2013). Formulation, characterization, and physicochemical evaluation of potassium citrate effervescent tablets. Advanced Pharmaceutical Bulletin, 3(1), 217. https://doi.org/10.5681/apb.2013.036
Aslani, A., & Jahangiri, H. (2013). Formulation, characterization, and physicochemical evaluation of ranitidine effervescent tablets. Advanced pharmaceutical bulletin, 3(2), 315. https://doi.org/10.5681/apb.2013.051
Banik, S., Karmakar, P., & Miah, M. A. H. (2015). Development and validation of a UV-spectrophotometric method for determination of vildagliptin and linagliptin in bulk and pharmaceutical dosage forms. Bangladesh Pharmaceutical Journal, 18(2), 163-168. https://doi.org/10.3329/bpj.v18i2.24316
Bhowmik, D., Chiranjib, B, Krishnakanth, Pankaj, R., & Chandira M. (2009). Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research, 163-177.
Bösenberg, L. H., & Van Zyl, D. G. (2008). The mechanism of action of oral anti-diabetic drugs: a review of recent literature. Journal of Endocrinology, Metabolism and Diabetes of South Africa, 13(3), 80-88. Https://doi.org/10.1080/22201009.2008.10872177
Harald, S. (2003). Effervescent dosage manufacturing. Pharmaceutical Technology Europe, 25-28.
Isaac, J., Kaity, S., Ganguly, S., (2013). Microwave‐induced solid dispersion technology to improve bioavailability of Glipizide. J Pharm Pharmcol, 65(2), 219-229. https://doi.org/10.1111/j.2042-7158.2012.01595.x
Jamzad, S., & Fassihi, R. (2006). Development of a controlled release low dose class II drug-Glipizide. International Journal of Pharmaceutics, 312(1-2), 24-32. https://doi.org/10.1016/j.ijpharm.2005.12.037
Josep, M., Suñé-Negre, Pérez-Lozano, P., Roig, M., Fuster, R., …Ticó, J. R. (2011). Optimization of parameters of the SeDeM Diagram Expert System: Hausner index (IH) and relative humidity (%RH), European Journal of Pharmaceutics and Biopharmaceutics, 79(2), 464-472. https://doi.org/10.1016/j.ejpb.2011.04.002
Kaleem, M. A., Alam, M. Z., Khan, M., Jaffery, S. H. I., & Rashid, B. (2020). An experimental investigation on accuracy of Hausner Ratio and Carr Index of powders in additive manufacturing processes. Metal Powder Report. https://doi.org/10.1016/j.mprp.2020.06.061
Khan, A., Iqbal, Z., Rehman, Z., Nasir, F., Khan, A., Ismail, M., & Mohammad, A. (2014). Application of SeDeM Expert system in formulation development of effervescent tablets by direct compression. Saudi Pharmaceutical Journal, 22(5), 433-444. https://doi.org/10.1016/j.jsps.2013.07.002
Kristensen, H. G., & Schaefer, T. (1987). Granulation: A review on pharmaceutical wet-granulation. Drug Development and Industrial Pharmacy, 13(4-5), 803-872. https://doi.org/10.3109/03639048709105217
Lieberman, H. A., Lachman, L., & Schwartz, J. B. (Eds.). (1980). Pharmaceutical dosage forms: Tablets (Vol. 1, pp. 109-124). M. Dekker.
Mohammed, K. A. B., Ibrahim, H. K., & Ghorab, M. M. (2016). Effervescent tablet formulation for enhanced patient compliance and the therapeutic effect of risperidone. Drug Delivery, 23(1), 297-306. https://doi.org/10.3109/10717544.2014.912693
Nanjwade, B. K., Adichwal, S. A., Nanjwade, V. K., Gaikwad, K. R., Thakare, S. A., & Manvi, F. V. (2012). Development and evaluation of gastroretentive floating tablets of Glipizide based on effervescent technology. J Drug Metab Toxicol, 3(3), 1-5. https://doi.org/10.4172/2157-7609.1000121
Nauck, M., Meininger, G., Sheng, D. O., Terranella, L., Stein, P. P., & Sitagliptin Study 024 Group. (2007). Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, compared with the sulfonylurea, Glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double‐blind, non‐inferiority trial. Diabetes, Obesity and Metabolism, 9(2), 194-205. https://doi.org/10.1111/j.1463-1326.2006.00704.x
Nelson, E. (1955). Measurement of the repose angle of a tablet granulation. Journal of the American Pharmaceutical Association, 44(7), 435-437. https://doi.org/10.1002/jps.3030440714
Osei-Yeboah, F., Minglun Zhang, M., Feng Y., & Sun C. C. (2014), A Formulation Strategy for Solving the Overgranulation Problem in High Shear Wet Granulation, Journal of Pharmaceutical Sciences, 103(8), 2434-2440. https://doi.org/10.1002/jps.24066
Östman, J., Christenson, I., Jansson, B., & Weiner, L. (1981). The anti-diabetic effect and pharmacokinetic properties of Glipizide: comparison of a single dose with divided dose regime. Acta Medica Scandinavica, 210(1‐6), 173-180. https://doi.org/10.1111/j.0954-6820.1981.tb09796.x
Patel, J. K., Patel, R. P., Amin, A. F., & Patel, M. M. (2005). Formulation and evaluation of mucoadhesive glipizide microspheres. AAps PharmSciTech, 6(1), E49-E55. https://doi.org/10.1208/pt060110
Rajashree, M., Yellanki, S. K., Patil, B. R., & Manvi, F. V. (2010). Development and evaluation of floating matrix tablets of Riboflavin. International Journal of PharmTech Research, 2(2), 1439-1445.
Ramabargavi, J. L., Pochaiah, B., Meher, C., Kishan, S., & Srujana, B. (2013). Formulation and in vitro evaluation of gastroretentive floating tablets of Glipizide. J Chem. Pharm Res, 5(2), 82-96.
Saleh, S. I., Boymond, C., & Stamm, A. (1988) Preparation of direct compressible effervescent components: spray-dried sodium bicarbonate. International Journal of Pharmaceutics, 45(1-2), 19-26. https://doi.org/10.1016/0378-5173(88)90030-0
Srinath, K. R., Chowdary, C. P., Palanisamy, P., Krishna, A., & Aparna, S. (2011). Formulation and evaluation of effervescent tablets of paracetamol. Int J Pharm Res Dev, 3(3), 76-104.
Thapa, P., Lee, A. R., Choi, D. H., & Jeong, S. H. (2017). Effects of moisture content and compression pressure of various deforming granules on the physical properties of tablets. Powder Technology, 310, 92-102. https://doi.org/10.1016/j.powtec.2017.01.021
Thoke, S. B., Sharma, Y. P., Rawat, S. S., & Nangude, S. L. (2013). Formulation development & evaluation of effervescent tablet of Alendronate sodium with vitamin D3. Journal of Drug Delivery and Therapeutics, 3(5), 65-74. https://doi.org/10.22270/jddt.v3i5.623
Tsume, Y., Langguth, P., & Garciaarieta, A. (2012). In silico prediction of drug dissolution and absorption with variation in intestinal pH for BCS class II weak acid drugs: Ibuprofen and ketoprofen. Biopharm Drug Dispos, 33(7), 366-377. https://doi.org/10.1002/bdd.1800
Verma, R. K., & Garg, S. (2005). Selection of excipients for extended release formulations of Glipizide through drug-excipient compatibility testing. J Pharmaceut Biomed, 38(4), 633-644. https://doi.org/10.1016/j.jpba.2005.02.026

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).









1.png)














