Evaluation of Corneal Reflectance in Sjögren's Syndrome Dry Eye
Abstract
Introduction: To measure corneal layers’ light reflectance (LR) in eyes affected by dry eye disease caused by Sjögren's syndrome (SSDE) with corneal confocal microscopy (IVCM) and to study the correlations with tear film tests.
Materials and Methods: Thirty-six patients affected by SSDE and 36 healthy subjects (HS) were enrolled in this retrospective study, participants of both groups were age and sex matched. Each study participants had a complete eye visit and break up time (BUT), Schirmer test, with and without stimulation, at the end of the visit an IVCM scan was performed. LR measured by IVCM at Bowman membrane (BM) level and at 50 µm, at 100 µm and at 200 µm deeper was compared in the two groups of participants. The correlations between LR measurements and tear film test results were investigated.
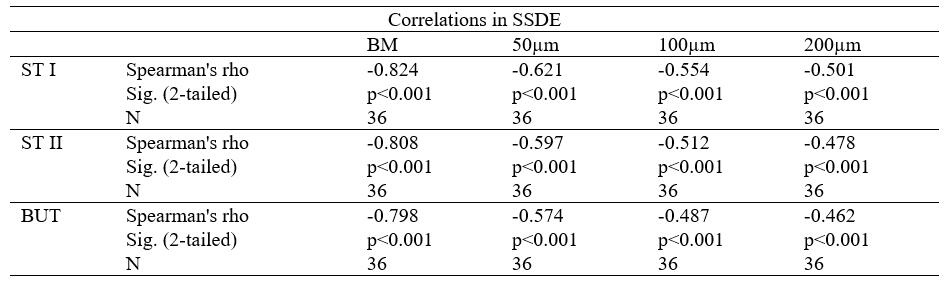
Results: In SSDE eyes, LR was significantly higher (p<0.001) in SSDE patients at BM level (+14.43 ± 3.27 LRU) and also in the other levels evaluated, compared with HP. Good correlations were observed between LR values at BM and Schirmer test ones with (r = -0.82) and without (r= -0.81) stimulation and BUT (r= 0.80) in SSDE eyes. Correlations values were Adecreasing the deeper corneal layers.
Conclusion: Even if need to be verified in further studies with a larger population, results obtained in this study suggest that IVCM could be an interesting and effective tool in evaluating the SSDE patients and it could be adopted by physicians’ community because it seems very promising.
References
[2] Afonso, A. A., Sobrin, L., Monroy, D. C. et al. (1999). Tear fluid gelatinase B activity correlates with IL-1a concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci., 40, 2506-2512.
[3] Pflugfelder, S. C., Jones, D., Ji, Z. et al. (1999). Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res., 19, 201-211.
[4] Solomon, A., Dursun, D., Liu Z. et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42, 2283-2292.
[5] The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf, 5, 75-92. https://doi.org/10.1016/s1542-0124(12)70081-2
[6] Tincani, A., Andreoli, L., Cavazzana, I. et al. (2013). Novel aspects of Sjögren's syndrome in 2012. BMC Med., 4, 93. https://doi.org/10.1186/1741-7015-11-93
[7] Stern, M. E., Beuerman, R. W., Fox, R. I. et al. (1998). The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea, 17, 584-589.
[8] Mathers, W. D. (1998). Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology., 100, 347-351.
[9] Villani, E., Galimberti, D., Viola, F. et al. (2007).The cornea in Sjogren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci., 48, 2017-2022.
[10] Tuisku, I. S., Konttinen, Y. T., Konttinen, L. M. et al. (2008). Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren's syndrome. Exp Eye Res., 86, 879-885. https://doi.org/10.1016/j.exer.2008.03.002
[11] Villani, E., Magnani, F., Viola, F. et al. (2013). In vivo confocal evaluation of the ocular surface
morpho-functional unit in dry eye. Optom Vis Sci., 90, 576-586. https://doi.org/10.1097/OPX.0b013e318294c184
[12] Labbé, A., Liang, Q., Wang, Z. et al. (2013). Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 54,5144-5150. https://doi.org/10.1167/iovs.13-12370
[13] Lanza, M., Iaccarino, S., Varricchi, G., D'Errico, T., Gironi, Carnevale U. A., Bifani, M. (2017). Corneal confocal microscopy alterations in Sjögren's syndrome dry eye. Acta Ophthalmol., 95, e366-e372. https://doi.org/10.1111/aos.13194
[14] Tuominen, I. S., Konttinen, Y. T., Vesaluoma, M. H. et al. (2003). Corneal innervation and morphology in primary Sjögren's syndrome. Invest Ophthalmol Vis Sci., 44, 2545-2549. https://doi.org/10.1167/iovs.02-1260
[15] Benítez, del Castillo, J. M., Wasfy, M. A., Fernandez, C. et al. (2004). An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci., 45, 3030-3035. https://doi.org/10.1167/iovs.04-0251
[16] Zhang, M., Chen, J., Luo, L. et al. (2005). Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea., 24, 818-824. https://doi.org/10.1097/01.ico.0000154402.01710.95
[17] Benítez, del Castillo, J. M., Acosta, M. C., Wassfi, M. A. et al. (2007). Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci., 48, 173-181. https://doi.org/10.1167/iovs.06-0127
[18] Villani E., Galimberti D., Viola F. et al. (2008). Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Invest Ophthalmol Vis Sci., 49, 560-564. https://doi.org/10.1167/iovs.07-0893
[19] Lin, H., Li, W., Dong, N. et al. (2010). Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci., 51, 122-128. https://doi.org/10.1167/iovs.09-3629
[20] Levy O., Labbé A., Borderie V., Hamiche T., Dupas B., Laroche L., Baudouin C., & Bouheraoua N. (2017). Increased corneal sub-basal nerve density in patients with Sjögren syndrome treated with topical cyclosporine A. Clin Exp Ophthalmol., 45, 455-463. https://doi.org/10.1111/ceo.12898.
[21] Lee, O. L., Tepelus, T. C., Huang, J., Irvine, A. G., Irvine, C., Chiu, G. B., & Sadda, S. R. (2018). Evaluation of the corneal epithelium in non-Sjögren's and Sjögren's dry eyes: an in vivo confocal microscopy study using HRT III RCM. BMC Ophthalmol, 18, 309. https://doi.org/10.1186/s12886-018-0971-3
[22] Cardigos, J., Barcelos, F., Carvalho, H., Hipólito, D., Crisóstomo, S., Vaz-Patto, J., & Alves, N. (2019). Tear Meniscus and Corneal Sub-basal Nerve Plexus Assessment in Primary Sjögren Syndrome and Sicca Syndrome Patients. Cornea., 38, 221-228. https://doi.org/10.1097/ICO.0000000000001800.
[23] Marchini, G., Mastropasqua, L., Pedrotti, E., Nubile, M., Ciancaglini, M., & Sbabo, A. (2006). Deep lamellar keratoplasty by intracorneal dissection: a prospective clinical and confocal microscopic study. Ophthalmology, 113, 1289-1300. https://doi.org/10.1016/j.ophtha.2006.01.071
[24] Morishige, N., Takahashi, N., Chikamoto, N., & Nishida, T. (2009). Quantitative evaluation of corneal epithelial oedema by confocal microscopy. Clin Experiment Ophthalmol, 37, 249-253. https://doi.org/10.1111/j.1442-9071.2009.02020.x
[25] Hillenaar, T., van Cleynenbreugel, H., Verjans, G. M., Wubbels, R. J., & Remeijer, L. (2012). Monitoring the inflammatory process in herpetic stromal keratitis: the role of in vivo confocal microscopy. Ophthalmology, 119, 102-1110. https://doi.org/10.1016/j.ophtha.2011.12.002
[26] Schiano-Lomoriello, D., Colabelli-Gisoldi, R. A., Nubile, M. et al. (2014). Descemetic and Predescemetic DALK in Keratoconus Patients: A Clinical and Confocal Perspective Study. BioMed Res Int., 123-156 https://doi.org/10.1155/2014/123156
[27] Shiboski, S. C., Shiboski, C. H., Criswell, L. et al. (2012). American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken)., 64, 475-487. https://doi.org/10.1002/acr.21591
[28] Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye Work Shop (2007). Ocul Surf, 5, 108-152. https://doi.org/10.1016/s1542-0124(12)70083-6
[29] McLaren, J. W., Nau, C. B., Patel, S. V. et al. (2017). Measuring corneal thickness with the ConfoScan 4 and z-ring adapter. Eye and Contact Lens, 33, 185-190. https://doi.org/10.1097/ICL.0b013e31802b3114
[30] McLaren, J. W., Bourne, W. M., & Patel, S. V. (2010). Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci., 51, 5610-5616. https://doi.org/10.1167/iovs.10-5614
[31] Labbe A., Alalwani H., Van Went C. et al. (2012). The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci., 53, 4926-4931. https://doi.org/10.1167/iovs.11-8708
[32] Mastropasqua, L., & Nubile, M. (2002). Confocal Microscopy of the Cornea. Thorofare(NJ),1, 127-131
[33] Tepelus, T. C., Chiu, G. B., Huang, J. et al. (2017). Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol, 255, 1771-1778. https://doi.org/10.1007/s00417-017-3680-3

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).









1.png)














