Hepatotoxicity and Histological Evaluation of Aqueous and Methanolic Leaf Extracts of Thaumatococcus Daniellii and Alchornea Cordifolia in Wistar Rat Models
Abstract
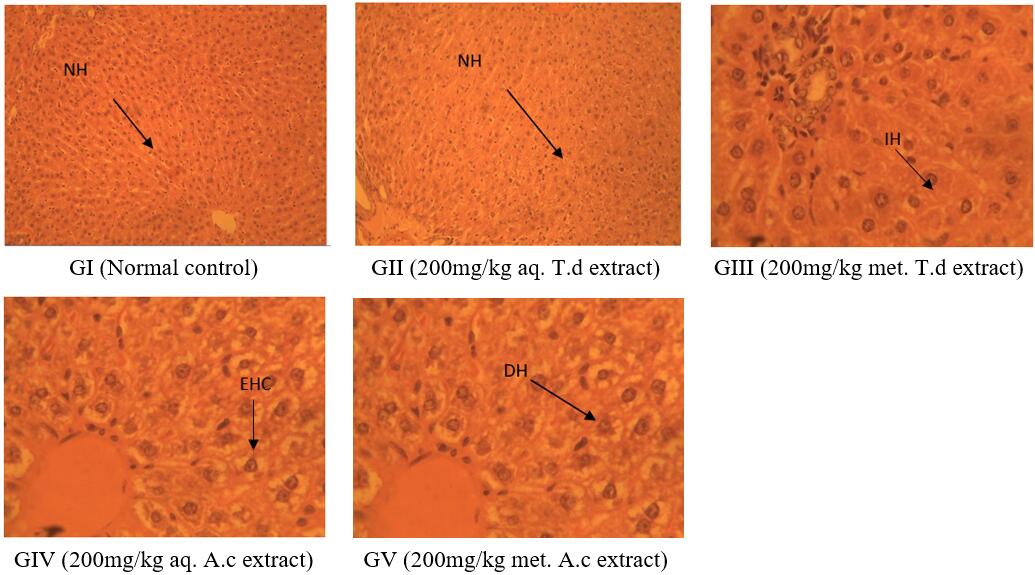
This study was carried out to ascertain the hepatotoxic potential of T.daniellii (T.d) and A. cordifolia (A.c). Investigations were conducted using standard methods. Oral administration of 200mg/kg aqueous leaf extracts of T.daniellii caused a non-significant increase in the activity of ALT (5.43±0.60IU/L), AST (16.93±0.26 IU/L) and ALP (160.70±1.04 IU/L) compared to the values recorded on the normal control (group I) ALT (3.84±0.16 IU/L), AST (14.19±0.52 IU/L) and ALP (157.26±0.64 IU/L). Group III administered with 200mg/kg methanolic leaf extract of T. daniellii manifested a significant elevation in the activity of ALT (13.15±0.89 IU/L), AST (22.84±0.38 IU/L) and ALP (170.40±0.44 IU/L) compared to the normal control. Similarly, groups IV and V which were orally administered with 200mg/kg aqueous and methanolic leaf extracts of A. cordifolia showed significant increase in the activity of ALT (6.32±0.33U/L), AST (17.70±0.030U/L) and ALP (161.13±0.09U/L) and ALT (7.55±0.59U/L), AST (19.35±0.26U/L) and ALP (165.38±0.35U/L) respectively compared to the values recorded on the control (group I). In conclusion, drug development protocols involving T. daniellii leaf should preferably use water as an ideal solvent. On the other hand, the hepatotoxicity associated with both aqueous and methanolic extracts of A. cordifolia could imply the presence of hepatotoxins in the leaf of the said plant.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).









1.png)














