The Crystal Structures and Cytotoxicity of Two Copper(II) Complexes Synthesized in a Reaction System
Abstract
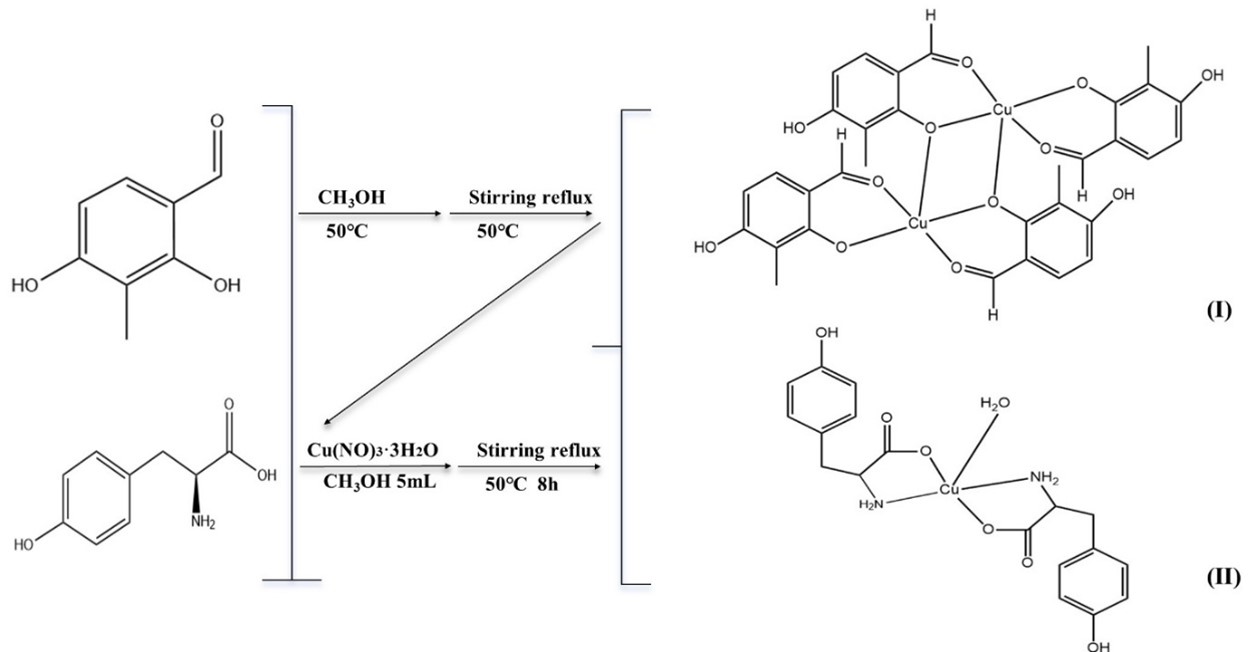
Breast cancer poses a serious threat to women's health. In this study, we obtained two different copper-based complexes (Ⅰ) and (Ⅱ) in a reaction system, and characterized their crystal structures using X-ray single crystal diffraction technology. Additionally, the cytotoxic effects of these two copper complexes on two breast cancer cell lines (MCF-7 and MDA-MB-231) ,non-cancerous human umbilical vein endothelial cells (HUVECs) and normal human liver cells (LO2) were evaluated using the MTT assay. The obtained results provided certain theoretical and practical foundations for the development of potential anti-cancer drugs.
References
[2] Ma, X., Dang, C., Kang, H., & Chen, J. (2015). Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-κB signalling pathways. International Immunopharmacology, 28(1), 399-408. https://doi.org/10.1016/j.intimp.2015.06.020
[3] Zhan, D., Ni, T., Wang, H., & Zhou, Z. (2022). Celastrol inhibits the proliferation and decreases drug resistance of cisplatin- resistant gastric cancer SGC7901/DDP cells. Anti-Cancer Agents in Medicinal Chemistry, 22(2), 270-279. https://doi.org/10.2174/1871520621666210528144006
[4] Yang, Z., Liu, Z., Ablise, M., & Yang, S. (2023). Design and synthesis of novel α-methylchalcone derivatives, anti-cervical cancer activity, and reversal of drug resistance in HeLa/DDP cells. Molecules, 28(23), 7697. https://doi.org/10.3390/molecules28237697
[5] Han, C., Liu, Z., Zhang, Y., Chen, J., Li, Y., Zhou, C., Wang, S., Zhang, S., Liu, M., Zhang, B., Xu, W., Chen, J., Wang, F., Shi, H., Wang, J., Yang, Y., & Dong, Y. (2020). Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nature Immunology, 21(5), 546-554. https://doi.org/10.1038/s41590-020-0641-5
[6] Liu, J., Liu, J., Guo, S. Y., Liu, C. M., & He, J. G. (2017). HSP70 inhibitor combined with cisplatin suppresses the cervical cancer proliferation in vitro and transplanted tumor growth: An experimental study. Asian Pacific Journal of Tropical Medicine, 10(2), 184-188. https://doi.org/10.1016/j.apjtm.2017.01.020
[7] Liu, H., Jiang, R., Lu, Y., Guo, Y., Zhang, W., Chen, X., Wang, Q., Liu, Q., Sun, Z., & Chen, X. (2022). Biodegradable amorphous copper iron tellurite promoting the utilization of Fenton-like ions for efficient synergistic cancer theranostics. ACS Applied Materials & Interfaces, 14(25), 28537-28547. https://doi.org/10.1021/acsami.2c03975
[8] Lomozová, Z., Hrubša, M., Conte, P. F., & Cvik, M. (2022). The effect of flavonoids on the reduction of cupric ions, the copper-driven Fenton reaction and copper-triggered haemolysis. Food Chemistry, 394, 133461. https://doi.org/10.1016/j.foodchem.2022.133461
[9] Jomova, K., Cvik, M., Lauro, P., & Valentová, J. (2023). The role of redox active copper(II) on antioxidant properties of the flavonoid baicalein: DNA protection under Cu(II)-Fenton reaction and Cu(II)-ascorbate system conditions. Journal of Inorganic Biochemistry, 245, 112244. https://doi.org/10.1016/j.jinorgbio.2023.112244
[10] Jiang, Y., Huo, Z., Qi, X., Zhang, H., Cui, Y., & Yin, Y. (2022). Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicine, 17(5), 303-324. https://doi.org/10.2217/nnm-2021-0374
[11] Copper(II) complexes with 2,2':6',2″-terpyridine derivatives displaying dimeric dichloro-μ-bridged crystal structure: Biological activities from 2D and 3D tumor spheroids to in vivo models. (n.d.). PubMed. Retrieved March 24, 2025, from https://pubmed.ncbi.nlm.nih.gov/36382103/
[12] Rodić, M. V., Leovac, V. M., Jovanović, L. S., Zmejkovski, B. B., & Vlahović, M. (2016). Synthesis, characterization, cytotoxicity and antiangiogenic activity of copper(II) complexes with 1-adamantoyl hydrazone bearing pyridine rings. European Journal of Medicinal Chemistry, 115, 75-81. https://doi.org/10.1016/j.ejmech.2016.03.003
[13] Draut, H., Rehm, T., Begemann, G., & Wiemann, T. (2017). Antiangiogenic and toxic effects of genistein, usnic acid, and their copper complexes in zebrafish embryos at different developmental stages. Chemistry & Biodiversity, 14(3). https://doi.org/10.1002/cbdv.201600302
[14] Zheng, H., Hu, C., Quan, Y., Song, C., Chen, S., Yu, X., Wang, Q., Zhang, X., & Liu, Q. (2023). A copper complex that combats triple negative breast cancer by restraining angiogenesis. Dalton Transactions, 52(22), 7626-7634. https://doi.org/10.1039/D3DT00738C
[15] da Silva, D. A., de Melo, A. C. C., Rodrigues, B. L., da Silva, A. M., de Oliveira, R. G. D., de Souza, B. G., Batista, A. A., & de Paula, E. (2022). Copper in tumors and the use of copper-based compounds in cancer treatment. Journal of Inorganic Biochemistry, 226, 111634. https://doi.org/10.1016/j.jinorgbio.2021.111634
[16] Liu, W. Q., Lin, W. R., Yan, L., Wu, D. B., Li, X. J., & Xu, J. (2024). Copper homeostasis and cuproptosis in cancer immunity and therapy. Immunological Reviews, 321(1), 211-227. https://doi.org/10.1111/imr.13276
[17] de Souza, Í. P., de Melo, A. C. C., Rodrigues, B. L., Santos, E. M. S., de Lima, M. E., de Castro, A. A. S., de Souza, B. G., & Batista, A. A. (2023). Antitumor copper(II) complexes with hydroxyanthraquinones and N,N-heterocyclic ligands. Journal of Inorganic Biochemistry, 241, 112121. https://doi.org/10.1016/j.jinorgbio.2023.112121
[18] Li, X., Chen, K., Lai, J., Lu, Z., Cao, Z., Yu, G., Li, S., Wang, H., & Liu, Y. (2024). Synthesis and antitumor activity of copper(II) complexes of imidazole derivatives. Journal of Inorganic Biochemistry, 260, 112690. https://doi.org/10.1016/j.jinorgbio.2024.112690
[19] Diz, M., Durán-Carril, M. L., Castro, J., & Bedia, J. C. (2022). Antitumor activity of copper(II) complexes with Schiff bases derived from N'-tosylbenzene-1,2-diamine. Journal of Inorganic Biochemistry, 236, 111975. https://doi.org/10.1016/j.jinorgbio.2022.111975
[20] Marzano, C., Pellei, M., Tisato, F., & Santini, C. (2009). Copper complexes as anticancer agents. Anti-Cancer Agents in Medicinal Chemistry, 9(2), 185-211. https://doi.org/10.2174/187152009787313837
[21] Miglioli, F., De Franco, M., Bartoli, J., Piacenza, L., Tosi, G., Gandin, V., & Marzano, C. (2024). Anticancer activity of new water-soluble sulfonated thiosemicarbazone copper(II) complexes targeting disulfide isomerase. European Journal of Medicinal Chemistry, 276, 116697. https://doi.org/10.1016/j.ejmech.2024.116697
[22] Jin, N., Zhang, L., & Qin, Y. X. (2025). Synthesis and crystal structure as well as cytotoxicity analysis of dichloro-terpyridine-copper complex. Modern Health Science, 8(2), 35-35. https://doi.org/10.30560/mhs.v8n2p35
[23] Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., & Puschmann, H. (2009). OLEX2: A complete structure solution, refinement and analysis program. Journal of Applied Crystallography, 42(2), 339-341. https://doi.org/10.1107/S0021889808042726
[24] Sheldrick, G. M. (2015). Crystal structure refinement with SHELXL. Acta Crystallographica Section C: Structural Chemistry, 71(1), 3-8. https://doi.org/10.1107/S2053229614024218

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).









1.png)














