Integration of Innovative Materials and Biotechnology in Joint Repair and Regeneration
Abstract
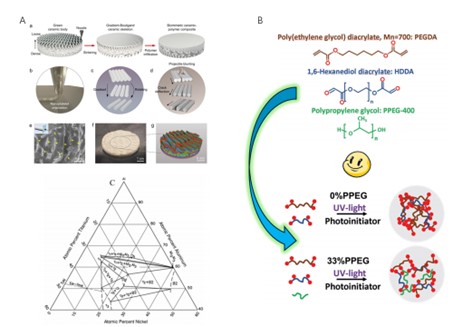
Musculoskeletal disorders, particularly osteoarthritis (OA), rheumatoid arthritis (RA), and post-traumatic osteoarthritis, are on the rise due to aging populations and increasing rates of obesity. The advent of regenerative medicine, particularly stem cell therapies, bioscaffolds, and growth factor delivery systems, has provided a new therapeutic direction for the treatment of musculoskeletal disorders by activating the body's self-repair mechanisms to restore joint structure and function. However, optimizing the integration, durability, and functional recovery of regenerated tissues remains a major challenge. To further address the potential of stem cell therapy, biomaterials, 3D bioprinting, and gene interventions in repairing and regenerating damaged joint tissues, this paper further traces the research advances of biomaterials, stem cell technologies, and gene therapies through in-depth analyses of relevant domestic and international studies and literatures, comprehensively evaluating their efficacy, safety, and potential clinical applications in joint repair and regeneration. At the same time, the paper focuses on the multidisciplinary integration of innovative materials and biotechnologies and the creative expansion from laboratory research to clinical applications. The role of nanotechnology, gene editing in orthopedics, and advances in 3D bioprinting in tissue engineering are also examined in some detail.It was found that mesenchymal stem cells (MSCs) show great potential for cartilage regeneration and inflammation reduction, and preliminary clinical trials have shown improvements in joint function and pain. Biomaterial-based strategies, such as collagen scaffolds combined with hydroxyapatite, have shown better results in repairing cartilage defects in animal models, which provides a low-cost, simple and environmentally friendly approach. In addition, nanotechnology and smart biomaterials are being explored for their potential for drug delivery and tissue repair, with nanocarriers protecting growth factors from degradation and enhancing targeted delivery. The convergence of innovative materials and technologies is bringing new therapeutic horizons to joint repair and regeneration. Multidisciplinary collaboration and the integration of innovative materials are opening up unprecedented therapeutic perspectives for joint repair and regeneration, and cross-disciplinary integration of materials science, regenerative medicine, and immunology is driving further development of precision medicine and personalized therapies. The integration of these technologies not only demonstrates great potential in the field of joint regeneration, but also provides new ideas for solving other complex biomedical problems. As these technologies continue to advance, the future of joint regenerative therapy is promising and will bring more benefits to patients with bone and joint diseases worldwide.
References
[2] Maniar, A. R., Luo, T. D., Somerville, L. E., MacDonald, S. J., Naudie, D. D. R., & McCalden, R. W. (2024). Minimum 15-year survival of a biconvex inlay patellar component in primary total knee arthroplasty: An analysis of 2,530 total knee arthroplasties from a single institution. Journal of Arthroplasty, 39(8S1), S80–S85. https://doi.org/10.1016/j.arth.2024.04.075
[3] Orita, K., Goto, K., Kuroda, Y., Kawai, T., Okuzu, Y., Takaoka, Y., & Matsuda, S. (2023). Long-term outcome of primary total hip arthroplasty with cementless bioactive glass ceramic bottom-coated implants and highly cross-linked polyethylene: A minimum 10-year analysis. Journal of Orthopaedic Science, 28(2), 385–390. https://doi.org/10.1016/j.jos.2021.12.019
[4] Cao, F., Xu, Z., Li, X. X., Fu, Z. Y., Han, R. Y., Zhang, J. L., Wang, P., Hou, S., & Pan, H. F. (2024). Trends and cross-country inequalities in the global burden of osteoarthritis, 1990–2019: A population-based study. Ageing Research Reviews, 99, 102382. https://doi.org/10.1016/j.arr.2024.102382
[5] Gao, Y., Zhang, Y., & Liu, X. (2024). Rheumatoid arthritis: Pathogenesis and therapeutic advances. MedComm (2020), 5(3), e509. https://doi.org/10.1002/adtp.202000133
[6] Punjwani, S., Jani, C., Liu, W., Kakoullis, L., Salciccioli, I., Al Omari, O., Merchant, A., Singh, H., Marshall, D., Shalhoub, J., Salciccioli, J. D., & Sehra, S. T. (2024). Burden of gout among different WHO regions, 1990–2019: Estimates from the global burden of disease study. Scientific Reports, 14(1), 15953. https://doi.org/10.1038/s41598-024-61616-z
[7] Chaudhary, H., Bohra, N., Syed, K., Donato, A., Murad, M. H., & Karmacharya, P. (2023). All-cause and cause-specific mortality in psoriatic arthritis and ankylosing spondylitis: A systematic review and meta-analysis. Arthritis Care & Research, 75(5), 1052–1065. https://doi.org/10.1002/acr.24820
[8] Chen, M., Liu, Y., Cao, Y., Zhao, C., Liu, Q., Li, N., Liu, Y., Cui, X., Liu, P., Liang, J., Fan, Y., Wang, Q., & Zhang, X. (2025). Remodeling the proinflammatory microenvironment in osteoarthritis through interleukin-1 beta tailored exosome cargo for inflammatory regulation and cartilage regeneration. ACS Nano, 19(4), 4924–4941. https://doi.org/10.1021/acsnano.4c16785
[9] Cui, S. H., Yan, Y., Lu, A., Dou, Y., Li, Z. W., Zhu, Z. H., Du, M. Z., Zhu, Y. F., Chen, X., Wang, X., Jiang, L. X., Shi, Y., Liu, X., Zhu, Y. J., Jiang, D., & Wang, J. C. (2024). Nanomedicines promote cartilage regeneration in osteoarthritis by synergistically enhancing chondrogenesis of mesenchymal stem cells and regulating inflammatory environment. ACS Nano, 18(11), 8125–8142. https://doi.org/10.1021/acsnano.3c11848
[10] Wang, M., Wu, Y., Li, G., Lin, Q., Zhang, W., Liu, H., & Su, J. (2024). Articular cartilage repair biomaterials: Strategies and applications. Materials Today Bio, 24, 100948. https://doi.org/10.1016/j.mtbio.2024.100948
[11] Abate, M., Paganelli, R., Pellegrino, R., Di Iorio, A., & Salini, V. (2024). Platelet rich plasma therapy in Achilles and patellar tendinopathies: Outcomes in subjects with diabetes (A retrospective case-control study). Journal of Clinical Medicine, 13(18), 5443. https://doi.org/10.3390/jcm13185443
[12] Suthar, M., Gupta, S., Bukhari, S., & Ponemone, V. (2017). Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. Journal of Biomedical Science, 24(1), 16. https://doi.org/10.1186/s12929-017-0324-1
[13] Kim, H. J., Park, J. M., Lee, S., Cho, H. B., Park, J. I., Kim, J. H., Park, J. S., & Park, K. H. (2022). Efficient CRISPR-Cas9-based knockdown of RUNX2 to induce chondrogenic differentiation of stem cells. Biomaterials Science, 10(2), 514–523. https://doi.org/10.1039/D1BM01716K
[14] Wei, X., Qiu, J., Lai, R., Wei, T., Lin, Z., Huang, S., Jiang, Y., Kuang, Z., Zeng, H., Gong, Y., Xie, X., Yang, J., Zhang, Y., Zhang, S., Zou, Z., Gao, X., & Bai, X. (2024). A human organoid drug screen identifies α2-adrenergic receptor signaling as a therapeutic target for cartilage regeneration. Cell Stem Cell, 31(12), 1813–1830.e8. https://doi.org/10.1016/j.stem.2024.09.001
[15] Peng, B. Y., Singh, A. K., Tsai, C. Y., Chan, C. H., Deng, Y. H., Wu, C. M., Chou, Y. R., Tsao, W., Wu, C. Y., & Deng, W. P. (2023). Platelet-derived biomaterial with hyaluronic acid alleviates temporomandibular joint osteoarthritis: Clinical trial from dish to human. Journal of Biomedical Science, 30(1), 77. https://doi.org/10.1186/s12929-023-00962-y
[16] Rui, J., Zhu, S., Xu, X., Wang, Y., Liu, Z., Cheng, G., Long, D., Cheng, L., & Dai, F. (2024, October 28). High-performance silk/polylactic acid composite scaffold material with immunomodulation and osteogenesis function. Materials Today Bio, 29, 101316. https://doi.org/10.1016/j.mtbio.2024.101316
[17] López-Serrano, C., Côté-Paradis, Y., Habenstein, B., Loquet, A., Le Coz, C., Ruel, J., Laroche, G., & Durrieu, M. C. (2024, July 31). Integrating mechanics and bioactivity: A detailed assessment of elasticity and viscoelasticity at different scales in 2D biofunctionalized PEGDA hydrogels for targeted bone regeneration. ACS Applied Materials & Interfaces, 16(30), 39165–39180. https://doi.org/10.1021/acsami.4c10755
[18] Platzer, H., Nees, T. A., Reiner, T., Tripel, E., Gantz, S., Hagmann, S., Moradi, B., & Rosshirt, N. (2020, April 28). Impact of mononuclear cell infiltration on chondrodestructive MMP/ADAMTS production in osteoarthritic knee joints—An ex vivo study. Journal of Clinical Medicine, 9(5), 1279. https://doi.org/10.3390/jcm9051279
[19] Adouni, M., Aydelik, H., Faisal, T. R., & Hajji, R. (2024, June 14). The effect of body weight on the knee joint biomechanics based on subject-specific finite element–musculoskeletal approach. Scientific Reports, 14(1), 13777. https://doi.org/10.1038/s41598-024-63745-x
[20] Chen, M., Liu, Y., Cao, Y., Zhao, C., Liu, Q., Li, N., Liu, Y., Cui, X., Liu, P., Liang, J., Fan, Y., Wang, Q., & Zhang, X. (2025, February 4). Remodeling the proinflammatory microenvironment in osteoarthritis through interleukin-1 beta tailored exosome cargo for inflammatory regulation and cartilage regeneration. ACS Nano, 19(4), 4924–4941. https://doi.org/10.1021/acsnano.4c16785
[21] Chou, C. H., Jain, V., Gibson, J., Attarian, D. E., Haraden, C. A., Yohn, C. B., Laberge, R. M., Gregory, S., & Kraus, V. B. (2020, July 2). Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Scientific Reports, 10(1), 10868. https://doi.org/10.1038/s41598-020-67730-y
[22] Hu, X., Li, Z., Ji, M., Lin, Y., Chen, Y., & Lu, J. (2022, September 29). Identification of cellular heterogeneity and immunogenicity of chondrocytes via single-cell RNA sequencing technique in human osteoarthritis. Frontiers in Pharmacology, 13, 1004766. https://doi.org/10.3389/fphar.2022.1004766
[23] Ji, Q., Zheng, Y., Zhang, G., Hu, Y., Fan, X., Hou, Y., Wen, L., Li, L., Xu, Y., Wang, Y., & Tang, F. (2019, January). Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Annals of the Rheumatic Diseases, 78(1), 100–110. https://doi.org/10.1136/annrheumdis-2017-212863
[24] Wang, X., Wang, C., Chu, C., Xue, F., Li, J., & Bai, J. (2024, May 17). Structure-function integrated biodegradable Mg/polymer composites: Design, manufacturing, properties, and biomedical applications. Bioactive Materials, 39, 74–105. https://doi.org/10.1016/j.bioactmat.2024.05.024
[25] Ajisafe, V. A., & Raichur, A. M. (2024, March 6). Snail mucus-enhanced adhesion of human chondrocytes on 3D porous agarose scaffolds. ACS Applied Materials & Interfaces, 16(9), 11324–11335. https://doi.org/10.1021/acsami.3c19557
[26] Mobbs, R. J., Parr, W. C. H., Huang, C., & Amin, T. (2022, June 18). Rapid personalised virtual planning and on-demand surgery for acute spinal trauma using 3D-printing, biomodelling and patient-specific implant manufacture. Journal of Personalized Medicine, 12(6), 997. https://doi.org/10.3390/jpm12060997
[27] Mitra, I., Bose, S., Dernell, W. S., Dasgupta, N., Eckstrand, C., Herrick, J., Yaszemski, M. J., Goodman, S. B., & Bandyopadhyay, A. (2021). 3D printing in alloy design to improve biocompatibility in metallic implants. Materials Today, 45, 20–34. https://doi.org/10.1016/j.mattod.2020.11.021
[28] Du, S., Huynh, T., Lu, Y. Z., Parker, B. J., Tham, S. K., Thissen, H., Martino, M. M., & Cameron, N. R. (2024, September 15). Bioactive polymer composite scaffolds fabricated from 3D printed negative molds enable bone formation and vascularization. Acta Biomaterialia, 186, 260–274. https://doi.org/10.1016/j.actbio.2024.07.038
[29] Liu, Z., Shi, J., Chen, L., He, X., Weng, Y., Zhang, X., Yang, D. P., & Yu, H. (2024, August). 3D printing of fish-scale derived hydroxyapatite/chitosan/PCL scaffold for bone tissue engineering. International Journal of Biological Macromolecules, 274(Pt 2), 133172. https://doi.org/10.1016/j.ijbiomac.2024.133172
[30] Singh, A. P., Rana, M., Pal, B., Datta, P., Majumder, S., & Roychowdhury, A. (2023, March). Patient-specific femoral implant design using metamaterials for improving load transfer at proximal-lateral region of the femur. Medical Engineering & Physics, 113, 103959. https://doi.org/10.1016/j.medengphy.2023.103959
[31] Leng, J., Sun, X., Zhang, W., Xu, J., Fu, X., Huang, J., Zhang, Y., Liu, Y., & Wei, Q. (2022, July 13). A personalized additively manufactured implant augments iliac crest autograft in canine segmental defect model. Biofabrication, 14(4), 045009.
[32] Lv, Y., Wang, B., Liu, G., & Wang, Q. (2024, June). Optimization design and experimental validation of the personalized porous implant for large segmental bone defect based on the mechanics–biology function. Materials & Design, 251, 111128.
[33] Wang, Y., Yuan, W., & Li, H. (2024, May). A novel 3D printed scaffold with superior swelling property and shape memory for treating bone defect. Journal of Materials Research and Technology, 31, 1706–1720.
[34] Zeng, W., Zhang, Y., Zheng, Y., Chen, Y., Yang, Y., Chen, C., & Wang, J. (2024, May). 3D-printed hierarchical bionic scaffolds promote vascularized bone regeneration in mandibular critical-sized defects. Bioactive Materials, 38, 307–320.
[35] Xu, W., Zhang, H., Liu, X., Hu, Y., Zhou, Y., & Li, L. (2024, July). Development of gelatin methacryloyl bioink with uniform chondrocyte distribution and enhanced extracellular matrix secretion. Carbohydrate Polymers, 324, 121512.
[36] Ekeuku, S. O., Yap, C. Y., Shavandi, A., & Lee, M. H. (2021, May). 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Journal of 3D Printing in Medicine, 5(3), 163–183.
[37] Lee, H., Han, W., Kim, H., Ha, D. H., Jang, J., & Cho, D. W. (2019, September). Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication, 11(4), 045025.
[38] Zhang, J., Geng, H., Kong, D., Lin, Z., Zhang, W., Zhang, Y., Lin, Y., & Yin, J. (2024, May 24). A printable bioink with cartilage-derived decellularized matrix and gelatin methacrylate for cartilage regeneration. Composites Part B: Engineering, 283, 116952.
[39] Liu, H., Xu, H., Zhang, C., Wang, C., Liu, Z., Tang, Q., & Zhao, L. (2022, February 22). Influence of particle size of decellularized extracellular matrix hydrogels on chondrocyte behavior and cartilage regeneration. Frontiers in Bioengineering and Biotechnology, 10, 818709.
[40] Sakai, S., & Kawakami, K. (2011, June). Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomaterialia, 7(7), 2898–2906.
[41] Chen, C. Y., Tsai, M. J., Huang, M. Y., & Tseng, Y. H. (2021, July). Sustained release of epigallocatechin gallate from chitosan–alginate composite sponges for cartilage regeneration. Colloids and Surfaces B: Biointerfaces, 203, 111763.
[42] Bakirci, E., Toprakhisar, B., Zeybek, M. C., & Ulubayram, K. (2020, April). Cartilage tissue engineering with chondroitin sulfate and oleuropein based hydrogels. International Journal of Biological Macromolecules, 150, 894–902.
[43] Olubamiji, A. D., Izadifar, Z., Si, J., Cooper, D. M., Eames, B. F., & Chen, D. X. (2016, December). Modulating mechanical behavior of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication, 8(4), 045020. https://doi.org/10.1088/1758-5090/8/2/025020
[44] Kang, H. W., Lee, S. J., Ko, I. K., Kengla, C., Yoo, J. J., & Atala, A. (2016, March 15). A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnology, 34, 312–319. https://doi.org/10.1038/nbt.3413
[45] Weng, T., Zhang, W., Xia, Y., Wu, P., Yang, M., Jin, R., Wang, Z., Liu, S., Cheng, K., Mao, C., & Xu, Y. (2021, May). 3D bioprinting for skin tissue engineering: Current status and perspectives. Journal of Tissue Engineering, 12, 1–18. https://doi.org/10.1177/20417314211028574
[46] Kim, B. S., Lee, J. S., Gao, G., & Cho, D. W. (2017, September 7). Direct 3D cell-printing of human skin with functional transwell system. Biofabrication, 9(2), 025034. https://doi.org/10.1088/1758-5090/aa71c8
[47] Vig, K., Chaudhari, A., Tripathi, S., Dixit, S., Sahu, R., Pillai, S., Dennis, V., Singh, S. R., & Dennis, V. A. (2017, November). Advances in skin regeneration using tissue engineering. International Journal of Molecular Sciences, 18(4), 789. https://doi.org/10.3390/ijms18040789
[48] Lee, V., Singh, G., Trasatti, J. P., Bjornsson, C., Xu, X., Tran, T. N., Yoo, S. S., Dai, G., & Karande, P. (2014, October). Design and fabrication of human skin by three-dimensional bioprinting. Tissue Engineering Part C: Methods, 20(6), 473–484. https://doi.org/10.1089/ten.tec.2013.0335
[49] Albanna, M., Binder, K. W., Murphy, S. V., Kim, J., Qasem, S. A., Zhao, W., Tan, J., & Jackson, J. D. (2019, January). In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Scientific Reports, 9, 1856. https://doi.org/10.1038/s41598-018-38366-w
[50] Abdollahiyan, P., Oroojalian, F., Mokhtarzadeh, A., & de la Guardia, M. (2020, May). Hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Biotechnology Journal, 15(12), 2000095. https://doi.org/10.1002/biot.202000095
[51] Chen, J., Han, Q., Pei, Y., Zhang, Y., & Wang, Y. (2021, November). Recent progress in 3D bioprinting for biomedical applications. Biotechnology Advances, 52, 107655.
[52] Mandrycky, C., Wang, Z., Kim, K., & Kim, D. H. (2016, June). 3D bioprinting for engineering complex tissues. Biotechnology Advances, 34(4), 422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011
[53] Lin, Y., & Zhou, T. (2020, August). Recent advances in 3D bioprinting technology for biomedical applications. Materials, 13(14), 3225.
[54] Dey, M., & Ozbolat, I. T. (2020, September). 3D bioprinting of cells, tissues and organs: Recent advances and future challenges. Current Opinion in Biotechnology, 65, 96–104. https://doi.org/10.1038/s41598-020-70086-y
[55] Gungor-Ozkerim, P. S., Inci, I., Zhang, Y. S., Khademhosseini, A., & Dokmeci, M. R. (2018, February). Bioinks for 3D bioprinting: An overview. Biomaterials Science, 6(5), 915–946. https://doi.org/10.1039/C7BM00765E
[56] Billiet, T., Vandenhaute, M., Schelfhout, J., Van Vlierberghe, S., & Dubruel, P. (2012, December). A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials, 33(26), 6020–6041. https://doi.org/10.1016/j.biomaterials.2012.04.050
[57] Hospodiuk, M., Dey, M., Sosnoski, D., & Ozbolat, I. T. (2017, January). The bioink: A comprehensive review on bioprintable materials. Biotechnology Advances, 35(2), 217–239. https://doi.org/10.1016/j.biotechadv.2016.12.006
[58] Murphy, S. V., & Atala, A. (2014, December). 3D bioprinting of tissues and organs. Nature Biotechnology, 32(8), 773–785. https://doi.org/10.1038/nbt.2958
[59] Ouyang, L., Highley, C. B., Sun, W., & Burdick, J. A. (2017, February). A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous inks. Advanced Materials, 29(8), 1604983. https://doi.org/10.1002/adma.201604983
[60] Malda, J., Visser, J., Melchels, F. P., Jüngst, T., Hennink, W. E., Dhert, W. J., Groll, J., & Hutmacher, D. W. (2013, December). 25th anniversary article: Engineering hydrogels for biofabrication. Advanced Materials, 25(36), 5011–5028. https://doi.org/10.1002/adma.201302042
[61] Kang, H. W., Lee, S. J., Ko, I. K., Kengla, C., Yoo, J. J., & Atala, A. (2016). A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnology, 34(3), 312–319. https://doi.org/10.1038/nbt.3413
[62] Schuurman, W., Khristov, V., Pot, M. W., van Weeren, P. R., Dhert, W. J. A., Malda, J. (2011). Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication, 3(2), 021001. https://doi.org/10.1088/1758-5082/3/2/021001
[63] Chimene, D., Lennox, K. K., Kaunas, R. R., & Gaharwar, A. K. (2016). Advanced bioinks for 3D printing: A materials science perspective. Annals of Biomedical Engineering, 44(6), 2090–2102. https://doi.org/10.1007/s10439-016-1638-y
[64] Kolesky, D. B., Truby, R. L., Gladman, A. S., Busbee, T. A., Homan, K. A., & Lewis, J. A. (2014). 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Advanced Materials, 26(19), 3124–3130. https://doi.org/10.1002/adma.201305506
[65] Cui, X., & Boland, T. (2009). Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials, 30(31), 6221–6227. https://doi.org/10.1016/j.biomaterials.2009.07.056
[66] Skardal, A., Zhang, J., & Prestwich, G. D. (2010). Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials, 31(24), 6173–6181. https://doi.org/10.1016/j.biomaterials.2010.04.045
[67] Grolman, J. M., Zhang, D., Smith, A. M., Moore, J. S., & Kilian, K. A. (2015). Rapid 3D extrusion of synthetic tumor microenvironments. Advanced Materials, 27(37), 5512–5517. https://doi.org/10.1002/adma.201501729
[68] Zhu, W., Qu, X., Zhu, J., Ma, X., Patel, S., Liu, J., Wang, P., Lai, C. S. E., Gou, M., Xu, Y., Zhang, K., & Chen, S. (2017). Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials, 124, 106–115. https://doi.org/10.1016/j.biomaterials.2017.01.042
[69] Zhang, Y. S., Yue, K., Aleman, J., Mollazadeh-Moghaddam, K., Bakht, S. M., Yang, J., Jia, W., Dell’Erba, V., Assawes, P., Shin, S. R., Dokmeci, M. R., Oklu, R., & Khademhosseini, A. (2017). 3D bioprinting for tissue and organ fabrication. Annals of Biomedical Engineering, 45(1), 148–163. https://doi.org/10.1007/s10439-016-1612-8
[70] Noor, N., Shapira, A., Edri, R., Gal, I., Wertheim, L., & Dvir, T. (2019). 3D printing of personalized thick and perfusable cardiac patches and hearts. Advanced Science, 6(11), 1900344. https://doi.org/10.1002/advs.201900344

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).









1.png)














