The Effect of Bio, Organic and Mineral Fertilizations on the Activity of the Urease, L-Glutaminase and L-Aspartase Enzymes in the Soil
Abstract
The study aimed to investigate the effect of the application of various sources of fertilizers on the activity of urease, L-glutaminase, and L-aspartase enzymes in the rhizosphere and bulk soils. Pots experiment was carried out in the wire canopy in one of the fields affiliated to the Faculty of Agriculture - the University of Qadisiyah for the summer season 2018 in soil with a silty loam texture. Seeds of mung bean crops were cultivated of a local variety (Khedrawi). The treatments of the study were two levels of nitrogenous mineral fertilizer (urea) (M1 and M2) (20 and 40) kg.N.h-1, respectively, a single level of organic fertilizer (poultry waste) (10 tons h-1), a single level of bio-fertilizer with Pseudomonas fluorescens bacteria, the control, and their interactions. The experiment was carried out according to Completely Randomized Design (C.R.D) with six replications. Means were compared according to the (L.S.D) test at the probability level (α=0.05(. The efficiency of urase, L-clotamene, and L-aspartase in the soil of the root zoon and bulk soil were estimated for all study parameters 30 days after planting. These measurements were done once again for some characteristics of vegetative and root growth after 60 days of planting.
The bio-fertilizer treatment (B) resulted in the highest increase in the efficiency of the urease enzyme and L-aspartase for 30 days of cultivation compared to the control treatment that recorded the least value of the enzyme activity average (45.22 and 42.54) µg N-NH4+.g-1 soil.2h-1, respectively, for the rhizosphere and bulk soils. These values were increased after 60 days of cultivation (45.34 and 43.16) µg N-NH4+.g-1 soil.2h-1, respectively, for the rhizosphere and bulk soils. This treatment achieves the highest increase in the activity of L-enzymes (25.89 and 24.72) µg N-NH4+.g-1 soil.2h-1, respectively, for the region of the rhizosphere and beyond. It increased after 60 days of cultivation (26.23 and 25.06) µg N-NH4+.g-1 soil.2h-1, respectively, for the region of the rhizosphere and bulk soils. The treatment of poultry wastes (O) resulted in the highest increase in the value of the activity of the L-clotamines enzyme in the rhizosphere and bulk soils for a period of 30 days from planting (43.47 and 57.39) µg N-NH4+.g-1 soil.2h-1, respectively. Its activity values then increased after 60 days of cultivation, reaching (44.33 and 40.43) µg N-NH4+.g-1 soil.2h-1, respectively.
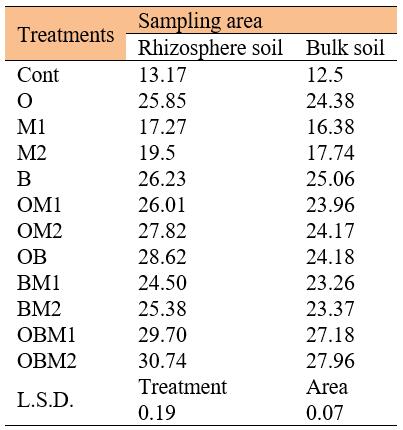
The overlap treatment between poultry residues and biofertilizers (OB) achieved the highest increase in the activity value for the urase enzyme, L-clotamines and L-aspartase in root zoon soil and distant soil for 30 days of cultivation (54.47, 46.84 and 28.28 µg N-NH4+.g-1 soil.2h-1, respectively, for the rhizosphere and (49.14, 37.81 and 23.94) µg N-NH4+.g-1 soil.2h-1, respectively. The activity of enzymes then increased after 60 days of cultivation (54.56, 47.69 and 28.62) µg N-NH4+.g-1 soil.2h-1, respectively, for the rhizosphere (49.23, 38.67 and 24.18) µg N-NH4+.g-1 soil.2h-1, respectively, for the bulk soil. The combination treatments between poultry residues, bio-fertilizer, and urea at level II (OBM2) achieved the highest increase in the activity values for urease enzyme, L-clotamines and L-aspartase in root zoon soil and bulk soil for 30 days of cultivation (58.46, 48.58 and 30.40) µg N-NH4+.g-1 soil.2h-1, respectively for the rhizosphere, and (54.13, 46.30 and 27.62) µg N-NH4+.g-1 soil.2h-1, respectively, for the bulk soil. The activity of enzymes then increased 60 days after planting (58.55, 49.44 and 30.74) µg N-NH4+.g-1 soil.2h-1, respectively, for the rhizosphere (54.22, 47.15 and 27.96) µg N-NH4+.g-1 soil.2h-1, respectively, outside the rhizosphere.
References
Abu, P., Falah, & Muhammad, S. Al-Shater (2011). Soil fertility and fertilization theoretical part. Publications of Damascus University, Faculty of Agriculture.
Aeschbacher, M. S., & Schwarzenbach, R. P. (2010). Novel Elec¬trochemical Approach To Assess The Redox Properties of Humic Acids. Environmental Science & Technology, 47, 87-93. https://doi.org/10.1021/es902627p
Ahmed, M. A., & El-Abagy, H. M. H. (2007). Effect of Bio-And Mineral Phosphorus Fertilizeron The Growth, Productivity And Nutritionalvalue Of Some Faba Bean (Vicia Faba, L) Cultivarsin Newly Cultivated Land. J. of Appl. Sci. Res., 3(6), 408-420.
Aljawasim, D. B., Khaeim, M. H., & Manshood, A. M. (2020). Assessment of arbuscular mycorrhizal fungi (Glomus spp.) as potential biocontrol agents against damping-off disease Rhizoctonia solani on cucumber. Journal of Crop Protection, 9(1), 141-147.
Al-Taweel, L. S. J. (2015). The effect of compost soil salinity on the microbial biomass and the effectiveness of some enzymes in the root zone of beans and tomatoes. Ph.D. thesis. Department of Soil and Water Resources _ College of Agriculture _ Baghdad University.
Al-Taweel, L. S., & Abo-Tabikh, M. M. (2019). Urea and Ammonium Sulfate Fertilizers and Humic Acid Effect on Urease Enzyme Activity in and out the Rhizosphere of Zea Mays L. Crop. Plant Archives, 19(1), 1905-1914.
Al-Wahaibi, M. bin H. (2008). Stimulated Ocean Root Bacteria for Plant Growth. Saudi Journal of Macroecological Sciences, 15(3).
Aziz, E. Eman, & El-Ashry, S. M. (2009). The efficiency of slow-release urea fertilizer on herb yield and essential oil Production of lemon balm (Melissa officinalis L.) plant. American-Eurasian J. Agric. And Environ Sci., 5(2), 141-147.
Barker, A. V., & Bryso, M. (2007). Nitrogen.In A.V. Barker, And D.J. Pilbeam, (Ed)" Handbook Of Plant Nutrition". CRC Taylor & Francis Group.
Bergstrom, D. W., & Monreal, C. M. (1998). Increased soil enzyme activities under two-row crops. Soil. Sci. Soc. Am. J., 62, 1295-1301. https://doi.org/10.2136/sssaj1998.03615995006200050021x
Black. C. A. (1965). Methods Of Soil Analysis Part (1). Physical Properties Am. Soc. Agron. INC. Publisher, Madison, Wisconsin, U.S.A. https://doi.org/10.2134/agronmonogr9.1
Brown. (2008). Functional And Structural Characterization Of Four Glutaminases From Escherichia Coli And Bacillus Subtilis. Biochemistry, 47(21), 5724-5735. https://doi.org/10.1021/bi800097h
Burns, R, G., El-Sayed, M. H., & McLaren, A. D. (1972a). Extraction of a urease-active organo-complex from the soil. Soil Biol. Biochem, 4,107-108. https://doi.org/10.1016/0038-0717(72)90048-X
Castellano, S. D., & Dick, R. P. (1991). Cropping and sulfur fertilization influence on sulfur transformation in soil. Soil Sci.Soc. Am. J., 54, 114-121. https://doi.org/10.2136/sssaj1991.03615995005500010020x
Coelho, E. F., Melo, D. M. D., Pereira, B. L., Santos, D. B. D., &Rosa, R. C. C. (2016). Roots Of ‘BRS Princesa’ Banana Fertigated With Humic Substances And Saponin-Based Plant Extracts. Acta Scientiarum. Agronomy Maringa, 38(4), 121-528, Oct.-Dec. https://doi.org/10.4025/actasciagron.v38i4.30790
Colombo, C., Palumbo, G., Sannion, F., & Gianfreda, L. (2002). Chemical And Biochemical Indicators Of Managed Agriculture Soils In:17th World Congress of Soil Science, Bangkok. Thailand. 17402, 1-9.
Conrad, J. P. (1940a). Catalytic activity causing the hydrolysis of urea in soils as influenced by several agronomic factors. Soil. Sci. Soc. Am. Proc., 5, 238-241. https://doi.org/10.2136/sssaj1941.036159950005000C0040x
Dodar, D. E., & Tabatabai, M. A. (2003). Effect of cropping systems on phosphates in soils. J. pland Nutr. Soil Sci., 166, 7-13. https://doi.org/10.1002/jpln.200390016
Frankenberger W. T. Jr., & Tabatabai, M. A. (199l) . Factors affecting L-glutaminase activity in soils. Biology and Fertility of Soils, 11, l-5. https://doi.org/10.1007/BF00335825
Gracia, C; T. Hernandez; F. Costa and B. Ceccanti. (1994). Biochemical parameters in soil regenerated by the addition of organic wastes. Waste Manage. Res., 12, 457-466. https://doi.org/10.1006/wmre.1994.1035
Hinsinger,P.; C. Plassard And B. Jaillarad .(2006). Rhizosphere: A New Frontier For Soil Biogeochemistry. Journal of Geochemical Exploration, 88, 210-213. https://doi.org/10.1016/j.gexplo.2005.08.041
Jarallah, Abbas Khudair Abbas. (1998). Biological transformation of urea fertilizer and its kinetic properties in salinity-affected soils. Master Thesis - College of Agriculture - University of Baghdad.
Jeber, B. A., & Hussein, M. K. (2019). "EFFECT OF FOLIAR APPLICATION OF AMINO ACIDS, ORGANIC ACIDS, AND NAPHTHALENE ACETIC ACID ON GROWTH AND YIELD TRAITS OF WHEAT." Plant Archives, 19(2), 824-826.
Jian, S., Li, J., Chen, J., Wang, G., Mayes, M.A., Dzantor, K. E., Hui, D., & Luo, Y., (2016). Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem, 101, 32-43. https://doi.org/10.1016/j.soilbio.2016.07.003
Juan, Y. H., Chen, L. J., Wu, Z. J., Wang, R., Sun, W. T., & Zhang, Y. L. (2010). Kinetic And Thermodynamic Behaviours of Soil Urease as Affected by Urease Inhibitors. R.C Suelo Nutr. Veg., 10(1), 1-11. https://doi.org/10.4067/S0718-27912010000100001
Kandeler, E., & Eder, G. (1993). Effect of cattle slurry in grassland on microbial biomass and on activities of various enzymes. Boil. Fertil. Soils, 16, 249-254. https://doi.org/10.1007/BF00369300
Katila, V., Lindsey, A. C., Mark, W. S., & Eduardo, A. R. (2013). Colonization Strategies of Pseudomonas Fluorescens Pf0-1: Activation Of Soil-Specific Genes Important for Diverse and Specific EnvironmentsBMC Microbiology, 13, 92. https://doi.org/10.1186/1471-2180-13-92
Khaeim, H. M. (2013). Mass selection with an optical sorter for head scab resistance in soft red winter wheat.
Khaeim, H. M., Clark, A., Pearson, T., & Van Sanford, D. (2019). Comparing Genetic Variation within Red Winter Wheat Populations with and without Image-Based Optical Sorter Selection. Al-Qadisiyah Journal For Agriculture Sciences (QJAS)(P-ISSN: 2077-5822, E-ISSN: 2617-1479), 9(2), 266-277. https://doi.org/10.33794/qjas.Vol9.Iss2.82
Khaeim, H. M., Clark, A., Pearson, T., & Van Sanford, D. (2019). Determining The Effect of Mass Selection for FHB Resistance in Soft Red Winter Wheat Using an Image-Based Optical Sorter. Al-Qadisiyah Journal for Agriculture Sciences (QJAS)(P-ISSN: 2077-5822, E-ISSN: 2617-1479), 9(2), 278-296. https://doi.org/10.33794/qjas.Vol9.Iss2.83
Khaeim, H. M., Clark, A., Pearson, T., & Van Sanford, D. (2019). Methods of Assessing Fusarium Damage to Wheat Kernels. Al-Qadisiyah Journal For Agriculture Sciences (QJAS)(P-ISSN: 2077-5822, E-ISSN: 2617-1479), 9(2), 297-308. https://doi.org/10.33794/qjas.Vol9.Iss2.91
Khaeim, H. M., Jeber, B. A., & Ali, M. A. (2019). Winter Wheat Genotypes Response to Different Water Quality. Int. J. Agricult. Stat. Sci., 15(2), 669-676.
Kuo, S., Sainju, U. M, & Jellum, E. J. (1997). Winter Cover Crop Effects On Soil Organic Carbon And Carbohydrate In Soil. Soil Sci. Soc. Am. J., 61, 145-152. https://doi.org/10.2136/sssaj1997.03615995006100010022x
Lakshmana, M. (2000). Azotobacter Inoculation And Crop Productivity In Azotobacter In Sustainable Agriculture: Ch. 11 (Ed.) Neeru Narula, India.
Lugtenberg, B.) 2006). Interactions In The Rhizosphere, Programs And Abstract Book, 7th International Workshop On Plant Growth Promoting Rhizobacteria Noodwijker Hout, The Netherlands. Lutgtenberg, Rubin. Leiden Univ.
Makinde, E. A., Ayeni, L. S., Jenny, S. O., & Odedina, J. N. (2010).Effect Of Organic, Organomineral, And NPK Fertilizer On Nutritional Quality Of Amaranthus In Lagos, Nigeria. Researcher, 2(2), 91-96.
Mittal, V., Singh, O., Nayyar, H., Kaur, J., & Tewari, R. (2008). Stimulatory Effect Of Phosphate Solubilizing Fungal Strains. (Aspergillus Awamori And Penicillium citrinum) on the Yield of Chickpea (Cicer Arietinum L.C.V. O. PF2) Soil Biochem, 40, 718-727. https://doi.org/10.1016/j.soilbio.2007.10.008
Nelson, E. B. (2004). Microbial Dynamics And Interaction In The Spermosphere. Annual Review of Phytopathology, 42, 271-309. https://doi.org/10.1146/annurev.phyto.42.121603.131041
Okabe, S., Nakamura, Y., & Satoh, H. (2012). Community Structure And In Situ Activity Of Nitrifying Bacteria In Phragmites Root-Associated Biofilms. Microbes Environ, 27(3), 242-249. https://doi.org/10.1264/jsme2.ME11314
Perucci, P., Gliusquiani, P. L., & Scaroni, L. (1982). Nitrogen losses from added urea and urease activity of a clay –loam soil amended with crop residues. Plant and Soil, 69, 457-463. https://doi.org/10.1007/BF02372466
Pinton, R., Varanini, Z., & Nannipieri, P. )2001). The Rhizosphere As A Site Of Biochemical Interaction Among Soil Components, Plants, And Microorganisms. In: The Rhizosphere: Biochemistry And Organic Substances At The Soil – Plant Interface. Pinton, R.; Z. Varanini And P. Nannipieri,(Eds.), Marcel Dekker, New York. Pp: 1-17.
Sahrawat, K. L. (1983). Relationship between soil urease activity and other properties of some tropical wetland rice soils. Fertilizer Research, 4, 145-150. https://doi.org/10.1007/BF01053251
Selim, E. M., Al-Neklawy, A. S., & Mosa, A. A. (2010). Humic Acid Fertigation of Drip Irrigated Cowpea Under Sandy Soil Condition.American. Eurasian J.Agric& Environ. Sci., 8(5), 538-543.
Shulka, G., & Varma, A. (2011). Soil Enzymology, Soil Biology 22.DOI, Springer-Verlag Berlin Heidelberg. https://doi.org/10.1007/978-3-642-14225-3
Sinsabaugh, R. L., Antibus, R. K., & Linkins, A. E. (1991). An Enzymic Approach to the Analysis of Microbial Activity During Plant Litter Decomposition. Agric Ecosyst Environ, 34, 43-54. https://doi.org/10.1016/0167-8809(91)90092-C
Sinsabaugh, R. L., Gallo, M. E., Lauber, C., Waldrop, M. P., & Zak, D. R. (2005). Extracellular Enzyme Activities And Soil Organic Matter Dynamics For Northern Hardwood Forests Receiving Simulated Nitrogen Deposition. Biogeochemistry, 75, 201-215. https://doi.org/10.1007/s10533-004-7112-1
Sparling, G. P. (1992). Ratio Of Microbial Biomass Carbon To Soil Organic Carbon As A Sensitive Indicator Of Changes In Soil Organic Matter. Aust J Soil Res., 30, 195-207. https://doi.org/10.1071/SR9920195
Tabatabi, M. A., & Bremner, J. M. (1972). Assay of urease activity in soils. Soil. Biol. Biochem., 4, 479-487. https://doi.org/10.1016/0038-0717(72)90064-8
Tietjen, T., & Wetzel, R. G. (2003). Extracellular Enzyme-Clay Mineral Complexes: Enzyme Adsorption, Alteration Of Enzymeactivity, And Protection From Photodegradation. Aquat. Ecol., 37(4), 331-339. https://doi.org/10.1023/B:AECO.0000007044.52801.6b
Vessey, J. K. (2003). Plant Growth Promoting Rhizobacteria As Biofertilizers. Plant And Soil, 255(2), 571-586. https://doi.org/10.1023/A:1026037216893
Wallenstein, M. E., Michael, N. W., & Zoppini, A. (2013). Soil Enzymes In A Changing Environment: Current Knowledge And Future Directions. Soil Biol Biochem, 58, 216-234. https://doi.org/10.1016/j.soilbio.2012.11.009
Yassin, M. F., Mahmoud, H. M. & Khamis, A. J. (2010). The Role of Organic Waste in Reducing the Effect of Salt Water on Some Chemical Soil and Readiness of N, P, and K. Iraqi Agricultural Science Journal, 41(1), 133-141.
Yoshimune. (2010). Crystal Structure of Salt-Tolerant Glutaminase From Micrococcus Luteus K-3 in the Presence and Absence of its Product L-Glutamate and its Activator Tris. FEBS Journal, 277(3), 738-748. https://doi.org/10.1111/j.1742-4658.2009.07523.x

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright for this article is retained by the author(s), with first publication rights granted to the journal.
This is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).








1.png)














